PETAH TIKVA, Israel, April 10, 2018 /PRNewswire/ — Leviticus Cardio, the inventor of wireless power transfer for Left Ventricular Assisted Devices, or “LVADs”, for patients with severe cardiac issues, has announced another round of successful animal trials, well beyond periods required to demonstrate suitability for chronic conditions. The company confirms that the successful studies put it in a strong position to advance its long-term strategy.
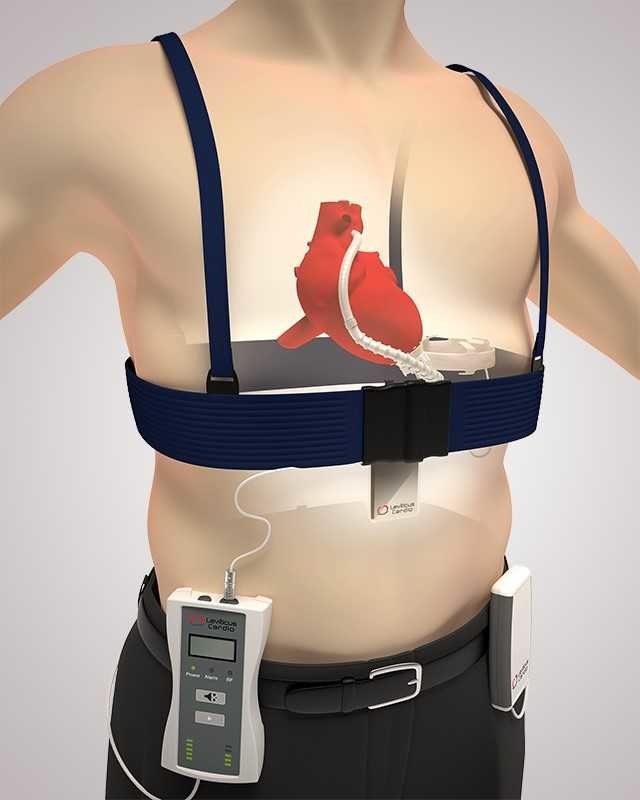
CET SYSTEM
LVADs are complex heart pumps for patients with significant cardiac muscle weakness. LVADs assist the patient’s heart for the duration of his life, and existing LVAD batteries require a powerline exiting the patient’s body (generally chest) for recharging.
Numerous experts have long viewed the driveline exiting a patient’s chest as the weak link in the LVAD architecture as it restricts patient lifestyle and is associated with high rates of infections, which can be lethal. Leviticus is the leader in wireless power transfer for LVADs which would eliminate the driveline.
In the latest study, conducted at the Catholic University of Leuven, Belgium, under the supervision of Professor Bart Meyns, MD, Ph.D., the technology has demonstrated efficiency, safety, and stability with repeated cycles of eight hours of discharge and recharge of an internal battery-powered mechanical circulatory support (MCS) device for more than 60 days.
Michael Zilbershlag, CEO of Leviticus Cardio comments, “This is the first step of an ambitious strategy. Our goal is to offer versatile wireless energy transfer to all patients requiring long term MCS support. Many key international opinion leaders have already expressed their willingness to support us and demonstrate the possibility of our CET technology.”
Professor Stephan Schueler, M.D., Ph.D., Newcastle upon Tyne, Freeman Hospital, UK, commented, “The successful completion of the animal trials with the wireless Coplanar Energy Transfer system opens a new era for long term LVAD implants.” Professor Jiri Maly, MD, Ph.D., from the Institute for Clinical and Experimental Medicine, Prague, stated that “based on our current experience, the CET system is easy to implant and doesn’t increase the complexity of surgery compared to a standard LVAD procedure.”
CALENDAR INFO
Leviticus will participate in the ISHLT 2018 (International Society for Heart & Lung Transplantation) meeting at The Acropolis Congress Centre, Nice, France. April 11th -13th, 2018, VAD update session.
To request a one-on-one meeting during the Congress please send a request to: [email protected].
GOT NEWS? click here
Google News, Bing News, Yahoo News, 200+ publications
About Leviticus Cardio
Leviticus Cardio (Leviticus-cardio.com) is a medical device company founded in 2008 and dedicated to improving the clinical outcome for patients implanted with a left ventricular assist device (LVAD) for impaired cardiac function. Major investors include The Trendlines Group, Israel’s foremost seed- and early-stage investment group, a consortium of acclaimed cardiovascular physicians, private investors and the Israel Innovation Authority of the Ministry of Economy.
Contact:
Michael Zilbershlag, CEO
T: +972-50-880-7135
[email protected]
Press Relations
Glenn Kenworthy
GlennKenworthy MarketingConsultancy
Tel: +44(0)1296-738-516
M: +44(0)7769-255-245
E: [email protected]
![]() View original content with multimedia:http://www.prnewswire.com/news-releases/leviticus-cardio-confirms-the-successful-completion-of-a-long-term-chronic-animal-study-300627185.html
View original content with multimedia:http://www.prnewswire.com/news-releases/leviticus-cardio-confirms-the-successful-completion-of-a-long-term-chronic-animal-study-300627185.html
SOURCE Leviticus Cardio






